OPSUMIT® (macitentan):
The only ERA with 7-year survival estimates1,2
These data are from long-term follow-up and an open-label extension study. These uncontrolled observations do not allow comparison with a group not given OPSUMIT® and cannot be used to determine the long-term effect of OPSUMIT® on mortality.
In long-term follow-up of patients who were treated with OPSUMIT® 10 mg in the placebo-controlled study (n=242), Kaplan-Meier estimates of survival at 1, 3, 5, and 7 years were 95%, 84%, 73%, and 63%, respectively. The median exposure to OPSUMIT® was 4.6 years.1,2
Overall survival Kaplan-Meier curve for patients randomized to OPSUMIT® 10 mg in SERAPHIN1,2
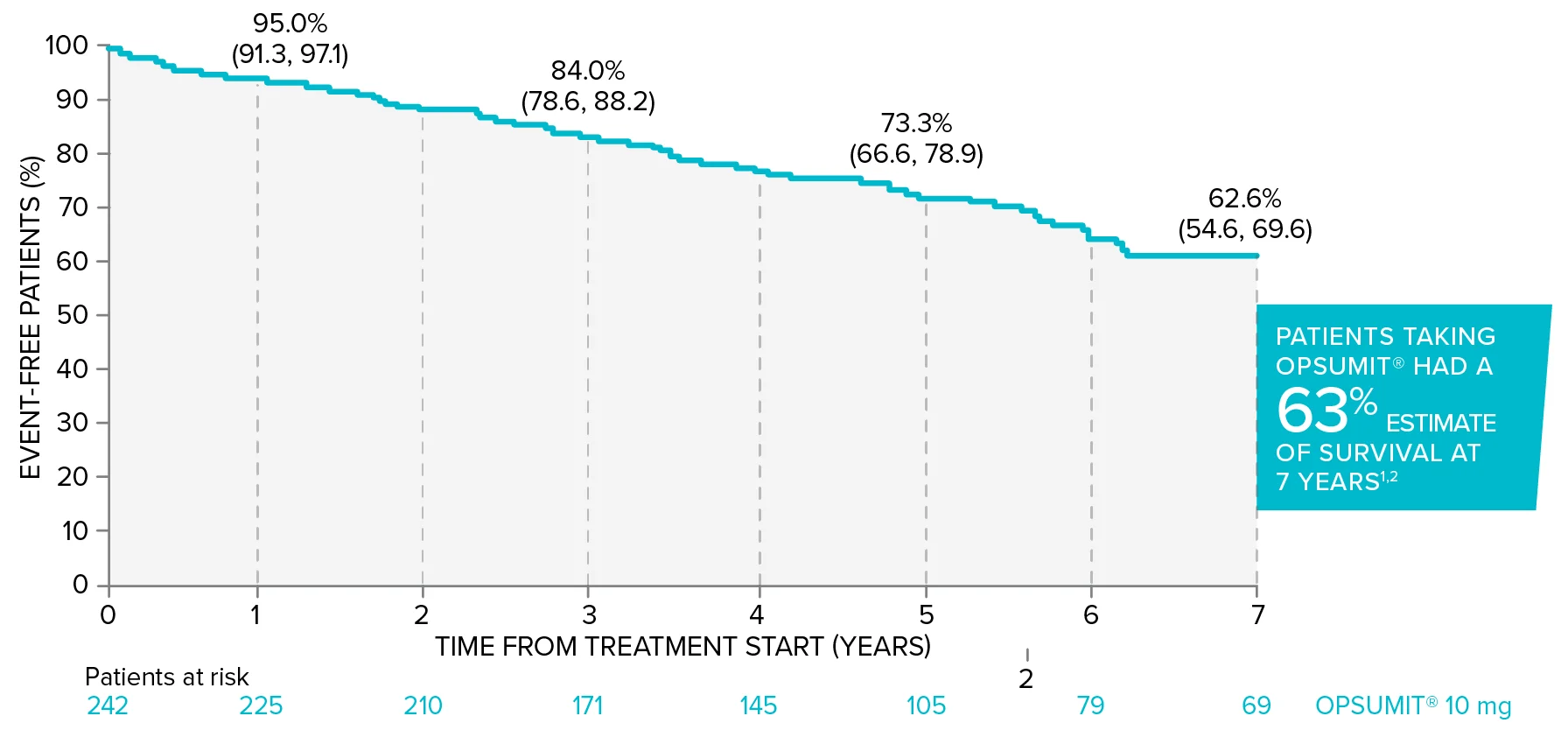
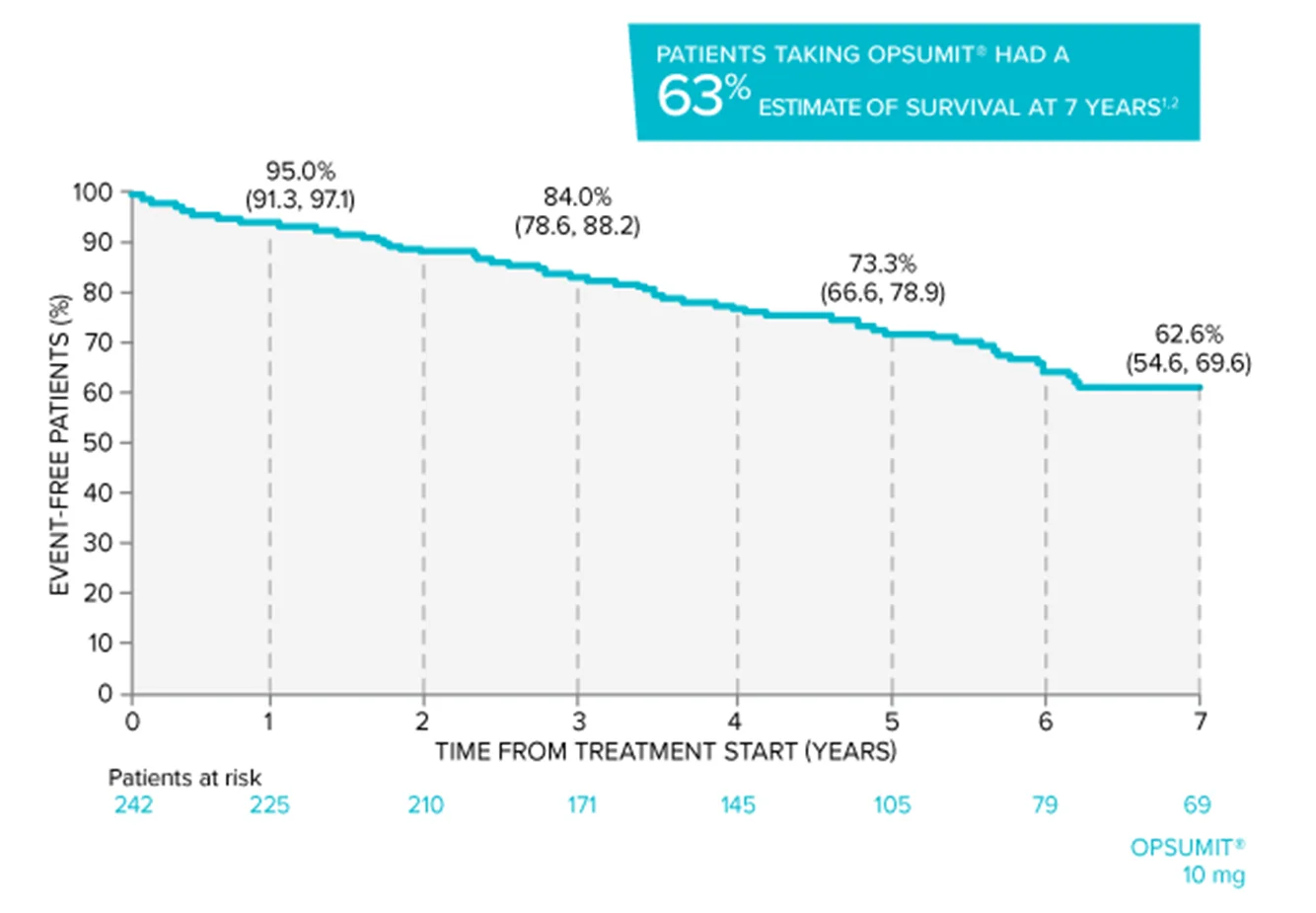
Adapted from Souza R, Delcroix M, Galié N, et al. Long-term safety, tolerability and survival in patients with pulmonary arterial hypertension treated with macitentan: results from the SERAPHIN open-label extension. Adv Ther. 2022;39(9):4374-4390. doi:10.1007/s12325-022-02199-x. Data presented are Kaplan-Meier survival estimates (95% CI).
Safety and Tolerability
VIEW DATASERAPHIN PAH-related hospitalization results
View the Data
CI=confidence interval;
ERA=endothelin receptor antagonist;
SERAPHIN=Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve CliNical Outcome.
References:
1. OPSUMIT® (macitentan) full Prescribing Information. Actelion Pharmaceuticals US, Inc.
2. Souza R, Delcroix M, Galié N, et al. Long-term safety, tolerability and survival in patients with pulmonary arterial hypertension treated with macitentan: results from the SERAPHIN open-label extension. Adv Ther. 2022;39(9):4374-4390.



